ToxStrategies’ scientists have extensive experience monitoring toxicology studies for domestic and international Contract Research Organizations (CROs). Our expert study monitors routinely help select appropriate CROs and facilitate protocol development, monitor important study events via site visits, problem solve issues and unexpected events, and review and interpret data and study reports to ensure fully GLP (Good Laboratory Practice) compliant, submission-ready regulatory data packages. Our scientists include former CRO staff with direct experience and knowledge of CRO processes and logistical requirements. This expertise enables us to effectively oversee both standard and complex study designs required for successful nonclinical programs.
During site visits, we perform a variety of activities, including verifying protocol directives are being followed and confirming that procedures are conducted according to facility standard operating procedures (SOPs). Supporting activities may include reviewing training files, SOPs, and regulatory audit inspection history, as well as observing CRO laboratory activities (e.g., formulations, analytical and bioanalytical, clinical pathology, histology, etc.). Importantly, we also collaborate with our CRO partners to reduce the impact of any GLP deviations or exceptions in order to minimize regulatory concerns.
We have experience monitoring studies employing standard rodent (e.g., mouse and rat) and non-rodent species (e.g., dog, nonhuman primate, minipig, and rabbit), as well as nonstandard species (e.g., immunocompromised/immunodeficient models, disease models, etc.).
Additional notable experience includes:
- On-site study monitoring in the United States, Canada, Europe, Japan, and China
- Extensive knowledge of GLPs (FDA, EMA, PMDA, NMPA, and OECD)
- Close working relationship with multiple leading nonclinical CROs
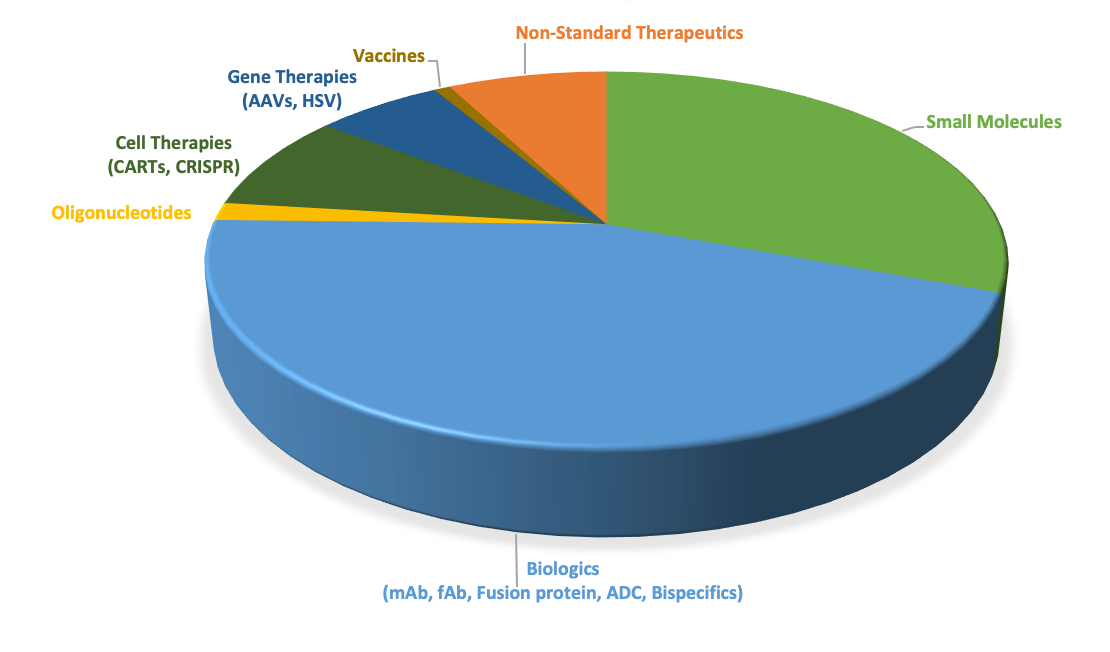
ToxStrategies’ therapeutic modality experience includes: